Industry Partners
The comprehensive, broad based nature of FoundationOne testing allowed us to be uniquely suited to provide reliable results across an unprecedented broad swath of predictive biomarkers in a clinically relevant turnaround time to attract multiple interested pharma partners with distinct therapeutic targets.
– Vincent Miller, M.D., Foundation Medicine
Lung-MAP is the National Cancer Institute’s (NCI’s) precision medicine platform trial in non-small cell lung cancer (NSCLC), a unique clinical trial that uses genetic screening to match patients to investigational new treatments.
Lung-MAP is also a mechanism for the NCI’s National Clinical Trials Network (NCTN) to collaborate with preferred research partners from industry.
The Lung-MAP platform offers a range of study options that facilitate testing novel agents or combinations in matched biomarker study designs and in non-matched studies in advanced NSCLC.
The trial also offers access to some of the world’s preeminent lung cancer researchers, with shared scientific leadership across the four NCTN groups focused on adult cancers – the Alliance, ECOG-ACRIN, NRG, and SWOG. This leadership team has built a highly effective working relationship with the U.S. Food and Drug Administration.
Lung-MAP operations are led by an experienced, dedicated team that relies on the operational and statistics and data management expertise of the SWOG Cancer Research Network within a unique long-term collaboration with the Foundation for the NIH and Friends of Cancer Research.
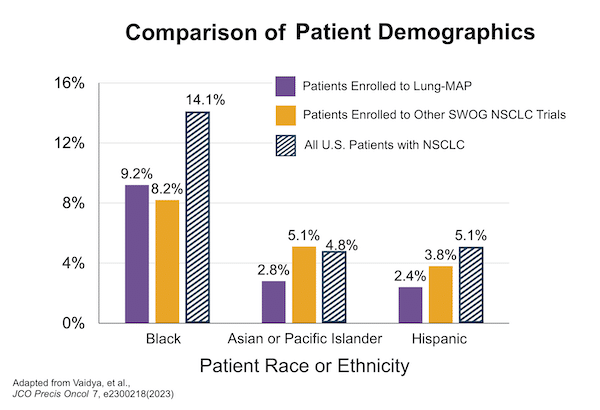
Lung-MAP’s broad reach across more than 800 network clinical sites nationwide, coupled with extensive engagement with patient advocacy communities, means the trial enrolls participants who are highly representative of the overall demographics of patients with advanced NSCLC in the U.S.
Benefits of Partnering with Lung-MAP
- Lung-MAP provides broad access to hundreds of academic and community-based clinical sites, with roughly one-half of enrollment coming from community providers.
- The trial offers access to leading lung cancer researchers and the FDA.
- As an NCTN trial, Lung-MAP provides access to all corners of the U.S. population and can help companies achieve the newly required trial goals for representative enrollment set by the FDA in June of 2024, as mandated by Congress in the Food and Drug Omnibus Reform Act.
- To ensure enrollment of a highly representative group of patients, the Lung-MAP team consistently works to
- take trials to where patients are treated
- ensure representative patient enrollment nationally across many dimensions, including socioeconomic, geographic location (e.g., rural vs urban), race, and ethnicity
- incorporate emerging FDA guidance
- relax trial eligibility criteria to enable enrollment of a broader range of patients
- The success of Lung-MAP’s approach to ensuring broadly representative enrollment has been documented in several recent publications, such as R. Vaidya JCO Precision Oncology 2023.
- As a trial conducted within the NCTN, Lung-MAP reduces the complications of individual contracting with sites.
- The trial’s drug distribution to participating sites is managed through the NCI.
- Lung-MAP’s well-organized trial development and management system means a streamlined timeline for protocol development.
- Lung-MAP builds on the NCTN’s infrastructure, offering opportunities for smaller companies that may have fewer resources.
- The trial collects valuable biospecimen resources and screening data that can be used in exploratory studies.
To learn more about partnering with Lung-MAP, contact LungMAPcontact@swog.org.
The comprehensive, broad based nature of FoundationOne testing allowed us to be uniquely suited to provide reliable results across an unprecedented broad swath of predictive biomarkers in a clinically relevant turnaround time to attract multiple interested pharma partners with distinct therapeutic targets.
– Vincent Miller, M.D., Foundation Medicine
