About Lung-MAP: Leadership
Leadership
This collaborative approach, with support from leading lung cancer advocacy organizations, helps to ensure that the needs of patients, clinicians, developers, and regulators are all considered in the design and operation of the trial.”
– Ellen Sigal, PhD, Chair and Founder, Friends of Cancer Research
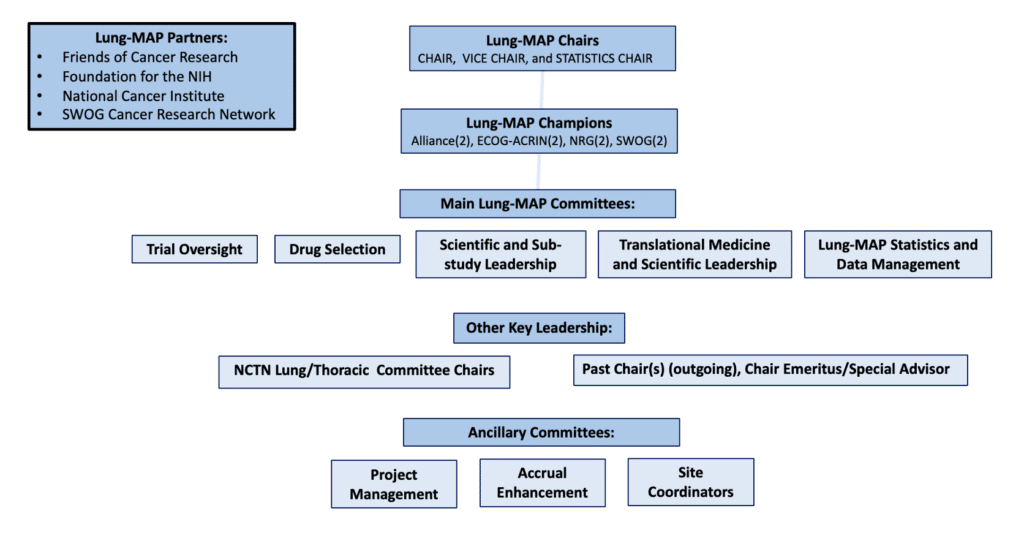
Lung-MAP represents a true partnership across all four of the adult NCI National Clinical Trials Network (NCTN) groups (Alliance, ECOG-ACRIN, NRG, and SWOG). Scientific leadership is distributed across the four groups and represents the depth and breadth of key opinion leaders in the NCTN. The study is scientifically led by the study chairs, medical oncology champions, and the committee chairs. The study is operationally overseen through a partnership between the NCTN groups, NCI/CTEP, Friends of Cancer Research (Friends), the Foundation for the National Institutes of Health (FNIH), and the SWOG Cancer Research Network. The study is also supported by ancillary committees (project management, accrual enhancement, site coordinators).
LUNG-MAP Study Chairs
- Chair: Karen Reckamp, MD (SWOG)
- Vice Chair: Saiama Waqar, MD (NRG)
- Statistical Chair: Mary Redman, PhD (SWOG)
Medical Oncology Champions
- Konstantin Dragnev, MD (Alliance)
- Martin Edelman, MD (NRG)
- David R. Gandara, MD (SWOG)
- Ticiana Leal, MD (ECOG-ACRIN)
- Joel Neal, MD, PhD (ECOG-ACRIN)
- Jyoti D Patel, MD (Alliance)
- Anjali Rohatgi, MD (NRG)
- John Wrangle, MD (SWOG)
Trial Oversight Committee Chairs
- Karen Reckamp, MD, (SWOG)
- Ellen Sigal, PhD, Friends of Cancer Research
- Vincent Miller, MD, EQRx
This committee includes NCI, FDA, and FNIH representatives and Advocates and PIs (ex-officio). It is governed by the Lung-MAP chair, FOCR, and a representative from an ex-officio company.
Drug Selection Committee Chair
- Konstantin Dragnev, MD (Alliance)
The internal Drug Selection Committee (DSC) has representatives from across the NCTN groups. The external DSC includes past sub-study chairs, NCTN group leads, and scientific experts.
Translational Medicine and Scientific Leadership Committee Chair
- Joel Neal, MD, PhD (ECOG-ACRIN)
This committee has representatives from across the NCTN with expertise in translational and clinical research.
Scientific & Sub-Study Leadership Committee Chair
- Corey Langer, MD (NRG)
This committee consists of Lung-MAP leadership, and sub-study champions, chairs, and co-chairs.
Lung-MAP Statistical and Data Management Committee (SWOG)
- Chair: Mary Redman, PhD
- Katie Minichiello, MS
- Michael Wu, PhD – TM Statistical Lead
- Louise Highleyman
- Kevin Moralda
Statisticians leading analyses from non-SWOG Lung-MAP sub-studies are also members of this group.
Imaging Chair
- Lawrence H. Schwartz, MD (SWOG/Alliance)
Lung-MAP Chair Emeritus and Senior Advisor
- Roy Herbst, MD, PhD (SWOG)
Lung-MAP Immediate Past Chair
- Hossein Borghaei (ECOG-ACRIN)
NCTN Group Lung/Thoracic Committee Chairs
- Jeff Bradley, MD (NRG)
- Julie R. Brahmer, MD (ECOG-ACRIN)
- Jhanelle E. Gray, MD (SWOG)
- Thomas Stinchcombe, MD (Alliance)
NIH/NCI Leadership
- Margaret Mooney, MD, Associate Director, Cancer Therapy Evaluation Program
- Shakun Malik, MD, Head of Thoracic Oncology Therapeutics, Cancer Therapy Evaluation Program
Friends of Cancer Research Leadership
- Ellen Sigal, PhD, Chairperson and Founder
- Jeff Allen, PhD, President and CEO
Foundation for the NIH Leadership
- Stacey Adam, PhD, Vice President, Science Partnerships
SWOG Cancer Research Network Leadership
- Charles D. Blanke, MD, Group Chair
- Michael LeBlanc, PhD, Group Statistician
Project Management
- Jennifer Beeler (SWOG)
- Gabe Chadwell (SWOG)
- Taqwa El-Hussein (FNIH)
- Crystal Miwa (SWOG)
- Jennifer Newsome, MS (FNIH)
Lung-MAP Site Coordinators Committee Co-Chairs
- Stephanie J. Reyes, RN, MSN, OCN, Georgia NCORP
- Maya Agosto, CRC , Georgia NCORP
This committee is composed of CRAs and nurses who represent the broad range of skills and expertise required for effective clinical trial administration and data management.
Lung-MAP Accrual Enhancement Committee Chair
- Ryan Hohman, JD, MPA, Friends of Cancer Research
This committee includes representatives from NCI, FNIH, FOCR, SWOG, and CCSA, and the chair of the Site Coordinators Committee.
This diverse, collaborative approach, with support from leading lung cancer advocacy organizations, helps to ensure that the needs of patients, clinicians, developers, and regulators are all considered in the design and operation of the trial.
– Ellen Sigal, Ph.D., Chair and Founder, Friends of Cancer Research
